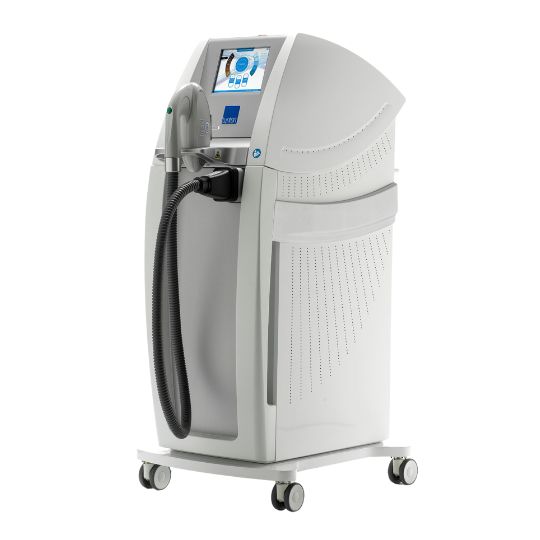
In a surprising move, Boston Scientific decided earlier this year to leave the CO₂ ENT surgical laser market by the end of 2024:
“Following a recommendation from the Israel-based laser franchise management team and additional careful considerations, Boston Scientific has made the strategic business decision to discontinue sales of its CO2 laser system portfolio in all global markets by the end of 2024. This is not a recall, and the decision is not due to concerns with safety or effectiveness. This decision is also not related to the Israel-Hamas war. We intend to continue to provide select CO2 service parts and single-use fibers and accessories, as well as service solutions, to support the provision of service to existing distributors and customers for up to seven years or per applicable contract terms, pending supply and other business constraints. Exact details of the portfolio discontinuation vary by country. Where appropriate, your local Boston Scientific representative will follow up with you separately to provide more information. We recommend not engaging in new tender applications for these products as we are unable to commit to the supply of inventory after 2024.”
Ref: Boston Scientific Announcement Jan 2024
By ceasing production of its Lumenis Acupulse and Ultrapulse DUO lasers, many healthcare providers are now grappling with the implications of this decision.
This blog post looks at some of the effects of Lumenis’ departure and offers some strategies for hospitals, healthcare providers, and procurement specialists to navigate the changing landscape of ENT laser technology effectively.
Understanding the Impact for ENT Surgical Lasers
Lumenis’ exit from the CO₂ ENT laser market has far-reaching consequences for medical institutions delivering ENT surgical procedures. Here’s a closer look at some of the key challenges ENT healthcare providers may face as a result:
-
Servicing Challenges
Obtaining servicing and support for your existing ENT laser is critical with precise surgical lasers. Service providers not only need experienced, knowledgeable and highly trained engineers but they should also have a fast and effective pipeline of approved spare parts and consumable items like laser fibres.
Any service provider without such resources may exhibit increases in wait times and cost for even simple servicing needs, both of which can have a significant impact on the level of support provided.
Whilst in the short term hospitals are unlikely to experience service issues for existing Acupulse and Ultrapulse DUO systems, it is highly likely to be a pressing concern for the future, particularly for those hospitals investing in new such lasers.
-
Maintenance Issues
Regular maintenance is crucial for the optimal performance of ENT lasers. However, with the announced discontinuation CO2 surgical lasers, ENT providers will increasingly struggle to find official replacement parts and expertise for repairs. Support options will become limited, which significantly increases the risk of disruption.
-
Replacement Parts
The availability of replacement parts is essential for maintaining the functionality of ENT lasers. However, sourcing parts may become increasingly difficult with Lumenis’ exit from the market (particularly if there are any business constraints) and turning to third-party vendors or aftermarket solutions cannot guarantee the same levels of reliability and safety.
Recommendations for Navigating the Change
Despite the challenges posed by the decision to discontinue the Acupulse and Ultrapulse DUO lasers, hospitals can take a variety of proactive steps to mitigate potential disruptions and reduce the risk of disruption to their ENT laser.
- Stock Up on Essential Parts
By forecasting usage and maintaining an inventory of components, ENT healthcare providers can minimise downtime caused by potential breakdowns.
- Look at Alternative Service Providers
Seeking out reputable third-party service companies can provide access to skilled technicians and urgent repair services for ENT lasers, at least in the short term.
However, third party service providers can be subject to unnecessary delays due to the lack of direct relationship with the device manufacturer.
- Upgrade Your Laser
Consider upgrading to newer, more advanced laser systems. This ensures access to cutting-edge technology, innovation, and robust replacement part and support services.
One such system is the DEKA SmartXide Trio, which will continue to be provided by Lynton Surgical in the UK. As an advanced laser platform capable of producing safe, repeatable, and efficient results, the SmartXide Trio offers power, flexibility, and precision.
- Consider Collaborative Solutions
Collaborating with other medical institutions or ENT specialty clinics for shared servicing and resource pooling may offer a temporary solution to extend the life of existing equipment in the short term.
The Reliability of DEKA ENT Surgical Lasers and Lynton Support

Investing in reliable alternatives like the DEKA SmartXide TRIO CO₂ surgical laser provides ENT healthcare providers with access to cutting-edge technology (such as state-of-the-art scanning equipment and robotic integration) and robust support services. With Lynton possessing the largest service and support team of its kind in the UK, they offer excellent aftersales support for which they have won numerous industry awards.
As DEKA’s representative in the UK, Lynton also benefits from a direct relationship with the laser manufacturer. As an authorised distributor, they have direct access to genuine parts, sourced from the manufacturer, which guarantees the authenticity and quality of disposable and replacement parts, ensuring optimal performance and longevity of your ENT laser.
Conclusion
Lumenis’ decision to exit the CO₂ ENT laser market presents significant challenges for ENT healthcare providers. However, by adopting a strategic approach that includes proactive planning and investment in alternative technologies, ENT clinics and hospitals can ensure operational continuity and continue to deliver high-quality patient care in the field of ear, nose, and throat surgery.
Learn More About the DEKA SmartXide Trio
If you are looking for help to navigate the upcoming changes, learn more about adding the Trio to your hospital by contacting Lynton today.