
The UK’s No.1 Laser & IPL ManufacturerCall today on: 01477 536 977
Menu
- Home
- Products
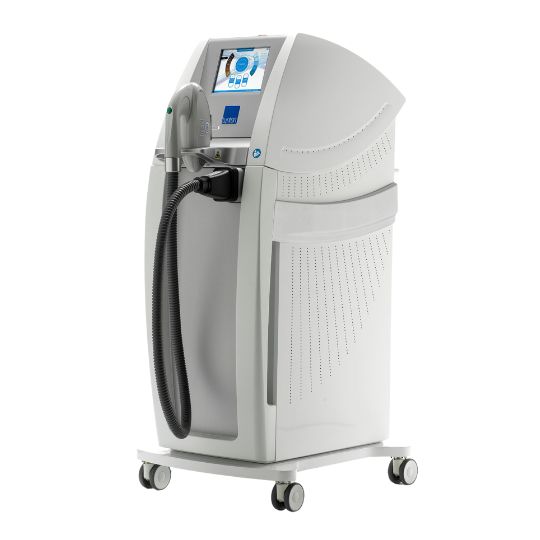 DYNAMIX®A powerful, all-in-one platform that treats a wide range of skin concerns, as well as hair and tattoo removal.
DYNAMIX®A powerful, all-in-one platform that treats a wide range of skin concerns, as well as hair and tattoo removal. EPN PenIntroduce EPN Pen to your business and increase your revenue by adding one of the most in-demand facial treatments to your portfolio.
EPN PenIntroduce EPN Pen to your business and increase your revenue by adding one of the most in-demand facial treatments to your portfolio.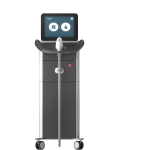 EVOLVEMedical Grade Diode Laser for Virtually ‘Pain-Free’ Hair Removal on All Skin Types
EVOLVEMedical Grade Diode Laser for Virtually ‘Pain-Free’ Hair Removal on All Skin Types Focus Dual®Radiofrequency Microneedling and High Intensity Focussed Ultrasound (HIFU) come together to give maximum results for retexturising and lifting the skin.
Focus Dual®Radiofrequency Microneedling and High Intensity Focussed Ultrasound (HIFU) come together to give maximum results for retexturising and lifting the skin. LUMINA®Upgrade as you grow, adding 24+ treatments to one professional Laser and IPL machine.
LUMINA®Upgrade as you grow, adding 24+ treatments to one professional Laser and IPL machine.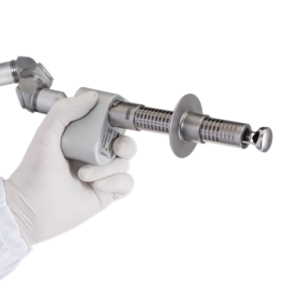 MonaLisa TouchAdvanced CO2 Laser Therapy for Intimate Care
MonaLisa TouchAdvanced CO2 Laser Therapy for Intimate Care Motus ProSafe, effective, and versatile – Motus Pro treats skin tones 1-6 with FDA-approved Alexandrite and ND:YAG laser technologies
Motus ProSafe, effective, and versatile – Motus Pro treats skin tones 1-6 with FDA-approved Alexandrite and ND:YAG laser technologies ONDAA revolutionary new non-surgical body sculpting machine like no other. Offering fat reduction, cellulite reduction, and skin tightening treatments with no downtime.
ONDAA revolutionary new non-surgical body sculpting machine like no other. Offering fat reduction, cellulite reduction, and skin tightening treatments with no downtime. Promax LipoRadio frequency and ultrasonic cavitation combined for body contouring and facial skin tightening.
Promax LipoRadio frequency and ultrasonic cavitation combined for body contouring and facial skin tightening. SmartPICOUltimate Skin Rejuvenation Nd:YAG Laser by DEKA
SmartPICOUltimate Skin Rejuvenation Nd:YAG Laser by DEKA SmartXide Punto CO2The latest gold-standard CO2 laser by Deka, with the ability to perform a broad range of treatments including CoolPeel™: CO2 but Cooler…
SmartXide Punto CO2The latest gold-standard CO2 laser by Deka, with the ability to perform a broad range of treatments including CoolPeel™: CO2 but Cooler… STUDIOPowerful and Compact Q-Switched Tattoo Removal Laser by DEKA.
STUDIOPowerful and Compact Q-Switched Tattoo Removal Laser by DEKA. Tetra ProPowered by the World’s Most Evidenced CO₂ Technology
Tetra ProPowered by the World’s Most Evidenced CO₂ Technology Lynton SkincareA unique skincare range formulated to work in synergy with any aesthetic treatment, enhancing treatment outcomes and long-term results.
Lynton SkincareA unique skincare range formulated to work in synergy with any aesthetic treatment, enhancing treatment outcomes and long-term results.
- Treatments
- Focus Dual Fusion
- illumismooth™ by Lynton
- SmartXide Bleph
- Acne Treatment
- Body Sculpting
- Cellulite Reduction
- CoolPeel
- Hair Removal: Laser & IPL
- HIFU
- illumifacial®
- Microneedling
- Nail Fungus
- Pigmentation Removal
- Scarring & Stretch Marks
- Skin Rejuvenation
- Skin Resurfacing
- Skin Tightening
- Tattoo Removal Lasers
- Vascular Lesions
- MonaLisa Touch
- Training
- Why Choose The Lynton Training Academy
- All Courses
- Level 3 Access to Aesthetic Therapies
- Core Of Knowledge
- Skin & Laser Applications in conjunction with The University of Manchester
- Level 4 Core Of Knowledge
- Level 4 Hair Reduction using Laser & IPL
- Level 4 HIFU Award
- Level 4 Skin Rejuvenation using Laser & IPL
- Level 4 Diploma Hair Reduction and Skin Rejuvenation – Laser & IPL
- Level 4 Skin Needling
- Level 5 Radiofrequency Microneedling
- Level 5 Laser Tattoo Removal
- Microneedling with Advanced Technology – Masterclass
- Fungal Nail, Verruca and Wart (Virtual) – Masterclass
- Laser Tattoo and Semi Permanent Makeup Removal – Masterclass
- Carbon Peel – Masterclass
- Advanced Clinical Update: Motus
- Advanced Clinical Update for Focus Dual
- Advanced Clinical Update for OEM Devices (Lumina, Excelight, 3JUVE and Dynamix)
- Advanced Clinical Update: Initia & Evolve
- Hair Removal Training
- Tattoo Removal Training
- Microneedling Training
- The Lynton Launch Programme
- Meet the Training Team
- Training FAQ’s
- Webinars
- The Lynton Group
- Why Us
- HIVE
- Resources
- In the Press
- Clinic Finder
- Contact Us



